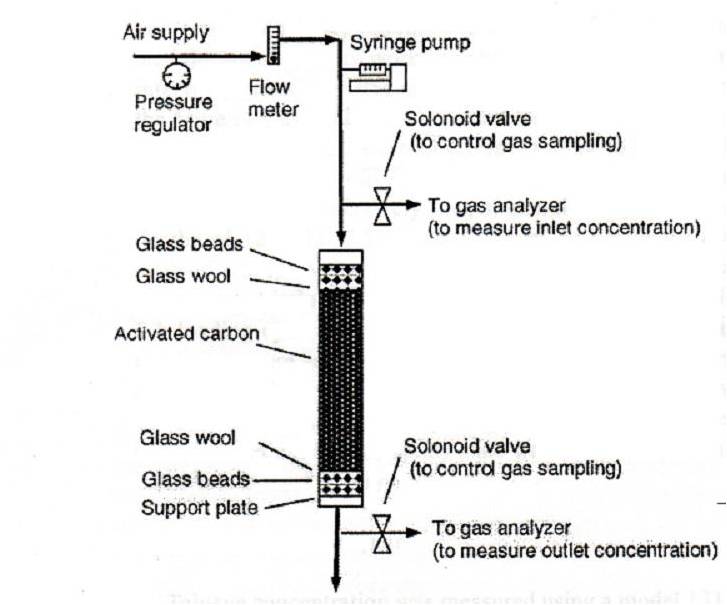
 GAC Filter
GAC Filter
Activated carbon is a natural material resulting from a mixture of bituminous coal, lignite, wood, coconut shell etc., activated by steam and other means, and each one have different adsorption properties. Activated carbon surface properties are both hydrophobic and oleophilic. This means that the carbon molecules dispel water but are attracted to oil. When flow conditions are suitable, dissolved chemicals in either liquid or gas flowing over the carbon surface adhere to the carbon in a thin film while the liquid or gas passes on. This process is called adsorption. Adsorption is a surface occurrence, in which molecules of adsorbate (toluene) are attracted and held to the surface of an adsorbent (carbon) until equilibrium is reached between adsorbed molecules and those still freely distributed in the carrying gas or liquid. This physical adsorption is a type of adsorption that is dependent mainly on surface attraction in which factors such as temperature, pressure and impurity may alter the equilibrium.As a result of the adsorption process, activated carbon is an effective method in removing organic compounds such as volatile organic compounds (carbon based VOC's). Other chemicals that activated carbon cannot reduce are non-carbon based anions (-) and cations (+) such as arsenic, fluorides, some heavy metals, nitrate, etc. The electronic forces, known as Van der Waal's forces, responsible for adsorption are related to those which cause like molecules to bind together, producing the phenomenon of condensation and surface tension. Van der Waal’s force refers to positive and negative forces between molecules such as dipole-dipole forces, dipole-induced dipole forces, as well as London forces. Some of the advantages of GAC vs other forms of carbon are: on a large scale such as municipal water treatment pools (gravity filters) for taste, odor and chemical reduction GAC is cheaper, very effective and can be re-used.

Just one gram of activated carbon has a surface area of approximately 500 m². This surface area is typically determined by nitrogen gas adsorption. This high surface area is due to extreme microporosity and macroporisity. These innumerable microscopic cracks and pores in the activated carbon, also known as microporisity, exponentially increases the surface area. While openings into the carbon structure may be of various shapes, the term pore, implying a cylindrical opening, is widely used. Microporosity is helpful in adsorbing lower molecular weight, lower boiling point organic vapors, as well as in removing trace organics in liquid or gas to non-detectable levels. Larger pore openings make up the macroporosity, which is useful in adsorbing very large molecules and aggregates of molecules, such as "color bodies" in raw sugar solutions. Activity level is often expressed as total surface area per unit weight, usually in square meters per gram. This total exposed surface will typically be in the range of 600-1200 m2/g. At the higher end of this range, one might better visualize one pound, about a quart in volume, of granular activated carbon with a total surface area of 125 acres. Granular vapor phase carbons were first widely used in WWI military gas masks. In the years between World Wars, this carbon was also used commercially in solvent recovery systems. Granular liquid phase carbons achieved their first prominent applications following WWI, in sugar de-colorization and in the purification of antibiotics. Today, there are hundreds of applications of both liquid and gas phase activated carbon.